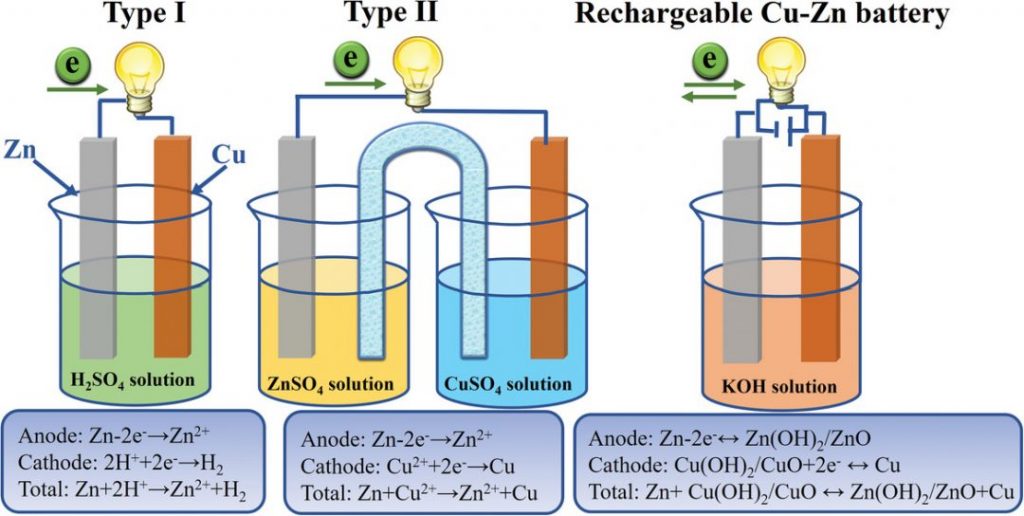
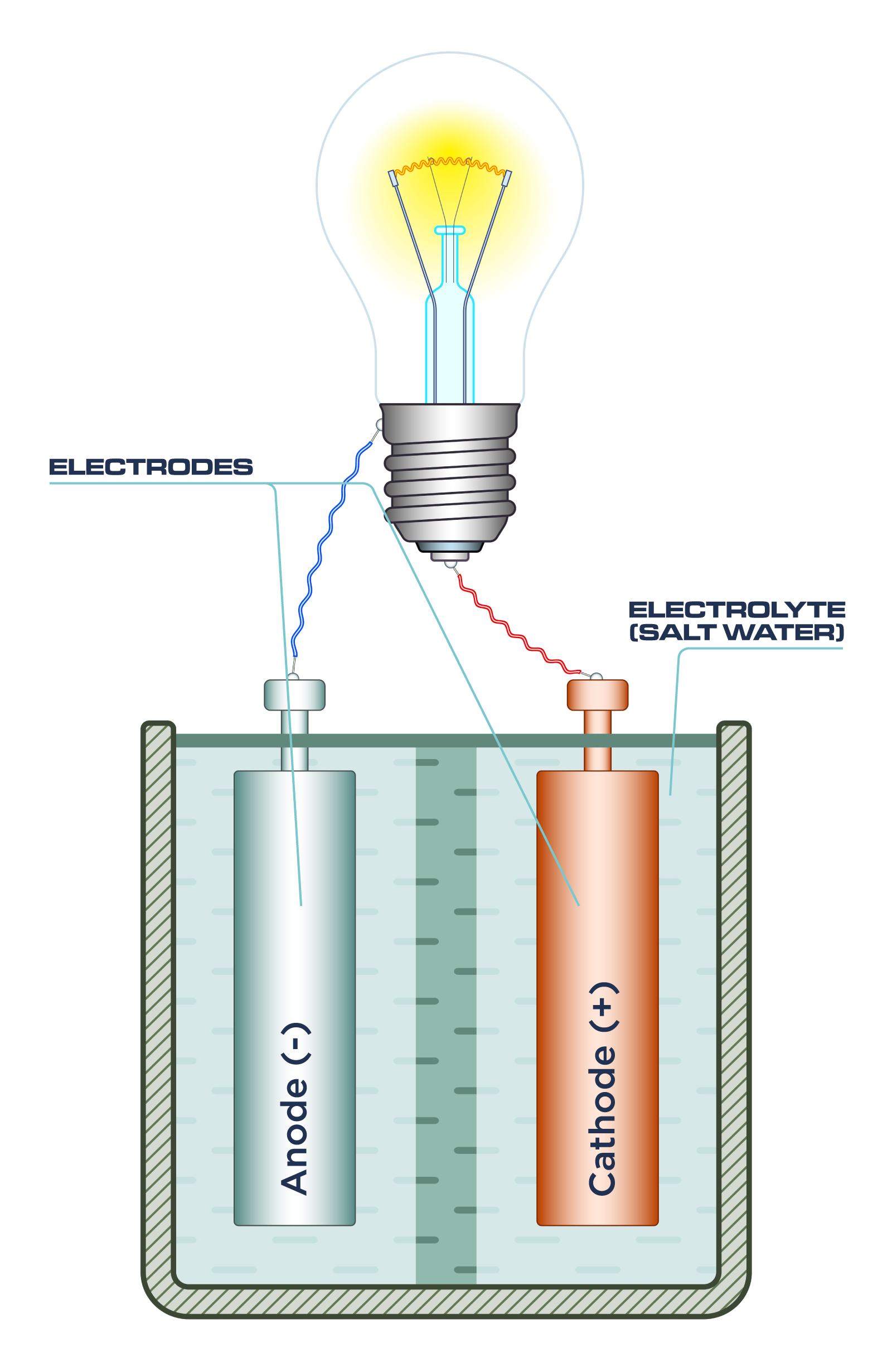
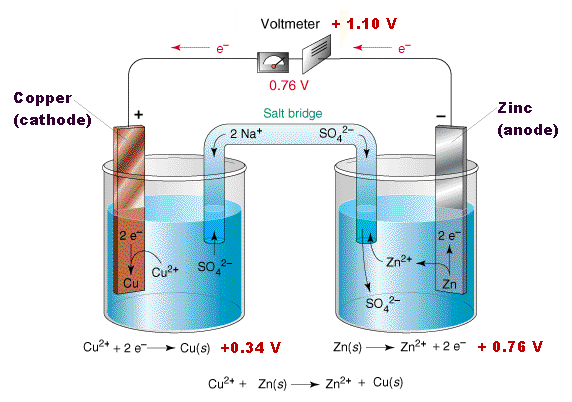
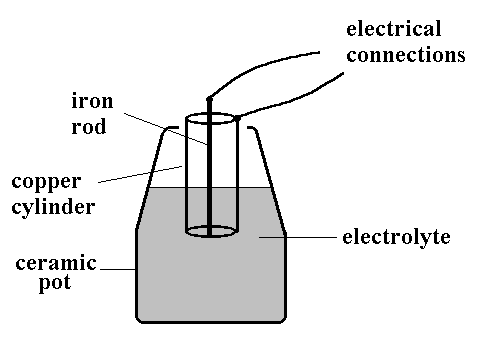
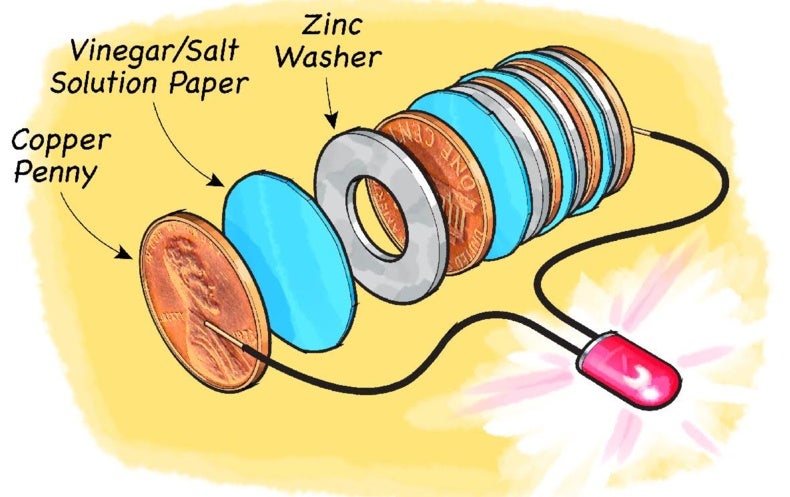
Amazing Homemade Project How to Make Saltwater Battery Using Iron, Copper - School Project. - YouTube
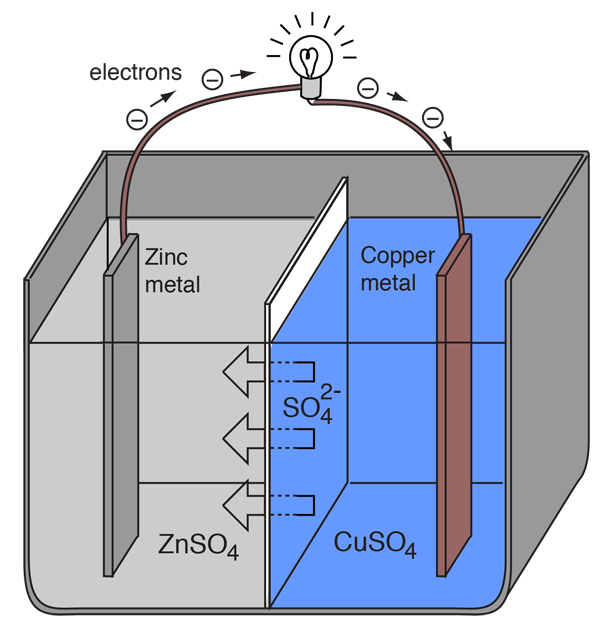
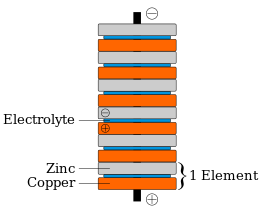
9 Diagram of a simple zinc-copper battery. Because zinc sulfate is more... | Download Scientific Diagram

VITSZEE Salt Water Battery DIY Science Experiment Complete kit | Copper, Zinc Plate (Strips), LED Light, wire | Science Lab| Fun-Magic Learning |Project | Practical | Science Fair | model | Tinkering

kit4curious salt water battery diy science experiment complete kit with instruction manual- Multi color : Amazon.in: Toys & Games

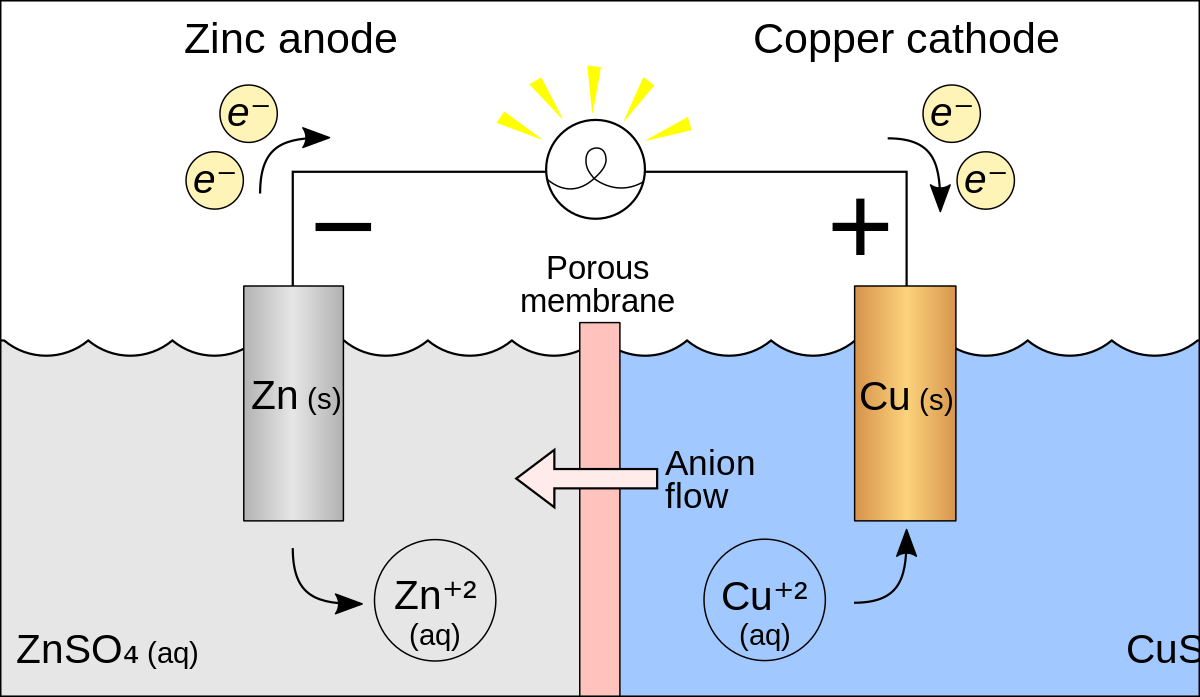
electrochemistry - What is the reaction between a copper anode and a zinc cathode? - Chemistry Stack Exchange